Ed eccoci alla penultima installazione (qui, qui, qui e qui le altre), dove andremo a vedere in qualche dettaglio il funzionamento dei recettori per l’amaro e per il pungente nel tratto gastrointestinale.
—————————————————————————————————————–
Coevolution and gut sensorium
Since the discovery of secretin, the first gut hormone, by Bayliss and Starling, the idea that the gastrointestinal tract had luminal chemo-sensitivity became a serious hypothesis, and since then it has become an integral part of the model of neurohumoral control of gastrointestinal function. It implies the idea that the gastrointestinal mucosa has a sensory role via tastant-sensing cells distributed in the gastric antrum or duodenal mucos. These cells can interact with luminal nutrients and release hormones in an endocrine or paracrine manner to transfer information about luminal nutrient content to other organs, including the brain, via endocrine or subepithelial neurons and vagal pathways.[1]
The gastrointestinal tract can thus be seen as a sense organ that has coevolved with some phytochemicals (nutrients, toxins, and stimulants such as bitter or pungent compounds), and which allows the detection of these phytochemicals and the appropriate response to them, like vomiting, aversion, regulation of appetite and satiety, alteration of stomach and intestinal motility and secretions. It also allows us to integrate this local information with higher level neurohumoral messages, and to command dietary, digestive, metabolic responses, and alarm responses in case of toxins.[2]
Much recent research has focused on identification of the cell types and specific receptors involved in this sensory mechanism. Particularly important are the enteroendocrine cells of the gastrointestinal tract.[3]

They act as primary chemoreceptors by releasing signaling molecules in response to changes in the luminal environment, showing at least two levels of action:
• transduction of luminal factors by release of signaling molecules, with paracrine actions on neighboring enterocytes, pivotal for digestion and food intake control.
• integration of sub-epithelial neurons, in particular receptors at afferent terminals in vagal and spinal pathways, providing an important link in the afferent transfer of information from gut surface to nervous system.
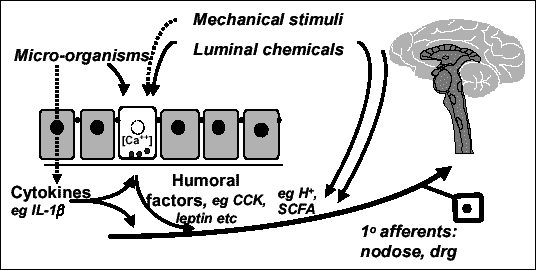
Enteroendocrine cells comprise cells producing cholecystokinin (CCK) (in duodenum and jejunum), glucagon-like peptide 1 (GLP-1) or peptide YY (PYY) (in ileum and colon), and also histamine and serotonin-producing[4] enterochromaffin-like cells.[5] These last ones are the predominant enteroendocrine cells, and they play a very important role in the regulation of gastrointestinal secretions, motility and visceral pain, mainly via serotonin secretion (involved in peristalsis, gastric motility and postprandial pancreatic secretion).[6]
These data give experimental support to the hypothesis that odor and taste are important clues for recognizing and classifying medicinal plants cross-culturally[7]. This hypothesis has been formalized by Shepard[8] when he proposed “sensory ecology” as a new theoretical framework for a cross-cultural understanding of sensation. “…[S]ensory ecology would be equally interested in cross-cultural variation and similarities and should incorporate physiological understandings and cultural constructions of sensory perceptions within a broad biocultural model addressing human-environment interactions”.
Let’s now have a look at the most important receptor families: bitter and pungent.
Bitter receptors
In Homo and in mammals, the capacity to detect the presence of toxic substances is strongly associated with the development of bitter receptors (taste receptor type 2 — TAS2R) in the oral cavity, an evolutionary-conserved mechanism to prevent ingestion of bitter-tasting compounds which are very often dietary toxins (e.g. alkaloids, saponines, etc.). This explains the fact that bitter is one of the few innate sensations universally recognized as disgusting and inducing aversive reactions.[9]
Bitter taste receptors were thought to have only gustatory functions, and to be limited to the oral cavity, but in the last 10 years there have been various reports of the presence of the receptors in extraoral sites, with non-gustatory functions. In particular TAS2Rs are well expressed in the gastrointestinal (endocrine cells in the mucosa of the gastric antrum and fundus, duodenum and gastroendocrine cells),[10] where they could regulate metabolic and digestive functions,[11] and possibly prevent the absorption of toxic compounds swallowed despite the bitter-detecting mechanisms in the mouth.[12]
While some TAS2R appear to be high selective, these are not the only ones linked to bitter sensations; there is evidence of less specific receptors that respond to relatively high concentrations of several unrelated bitter ligands, a mechanism which might explain how few receptors mediate the perception of numerous bitter compounds. This mechanis might have evolved to protect organisms from ingesting any of an enormous set of unrelated chemical potentially toxic compounds.
Some of the chemical characteristics of compounds which act as agonists at bitter receptors[13] are the presence of:
• phenyl-β-D-glucopyranosides (their affinity decreased approximately 200-fold when the phenyl group was replaced by a methyl moiety)
• glicoside formed by an aglycon attached to a gluco- or mannopyranose moiety in the β-glycosidic configuration.
• aglycons with one or two aromatic rings (stronger agonist properties than a methyl group)
• compounds containing an –N=C=S group.
Some of the secondary plant metabolites with agonist activity[14] are:
• aristolochic acid
• cyclohexamide
• naphthalaldehydic acid
• naphthoic acid
• nitrolnaphthalene
• papavarine
• picrotin
• picrotoxinin
• piperonylic acid
• quinacrine
• salicin
• strychnine
• α-thujone
Moving from the realm of molecules to that of plants, let us take as an example a very common weed, Dandelion (Taraxacum officinale). The sesquiterpene lactones of the eudesmanolide and germacranolide type unique to Taraxacum are intensely bitter; the elicited sensation of bitterness correlates well with the potentially poisonous nature of sesquiterpene lactones, often highly irritating for the nose and throat mucosa (hence the name sneezeweed given to many of the plants containing these substances) and cytotoxic. Although the lactones of the eudesmanolide type do not seem to posses toxic activity, they still elicit the bitterness response.
Effects of the stimulation of bitter receptors
The ingestion of relevant quantities of bitter substance will provoke a delay in gastric emptying in the subjects, followed by nausea and at times emesis.[15] Lower doses of bitters are still perceived as disgusting and this perception helps to fix the offensive food in the memory; the elicited chain of reactions is too weak to provoke emesis, but still strong enough to elicit a biliary stimulation.[16]
The activation of TAS2R in the gastrointestinal tract promotes the release of GI peptides via the protein gustducin – in particular CCK, and perhaps also PYY and GLP-1 – that can in turn activate neural reflexes.[17]
The stimulation of CCK secretion in turn triggers the release of digestive enzymes from the pancreas and the emptying of bile salts from the gallbladder into the duodenum, which then induce protein and fat digestion. CCK release also regulates gastrointestinal motility, and gastric acid secretion, induces reflex inhibition of gastric emptying (although it does not significantly impair antral motility),[18] and causes satiation by stimulating vagal afferent endings by activating CCK-1 or CCK-A receptors.[19]
Since CKK secretion is also influenced by a transcription factor (SRBEP) that is a key regulator of lipid metabolism, and since a diet rich in vegetables is usually both low in cholesterol and higher than normal in bitter substances, CKK activity seems aimed at reducing the absorption of the bitter compounds (reduced appetite, delayed gastric emptying) and of maximizing the absorption of complex carbohydrates and of low levels of essential fatty acids and fat-soluble vitamins (stimulation of gall-bladder contractions, increase in bile acid excretion).[20]
Stimulation of these receptors seems also to modulate glucose homeostasis, helping in the regulation of glucose and insulin levels.[21]
Pungent receptors
Pungency, like bitterness, is universally recognized as aversive, causing sensations of burning and pain.
A set of ion channels (Transient Receptor Potential channels — TRP channels) expressed in the gut responds to a varied class of pungent compounds. It includes the vanilloid channel TRPV1 which responds to capsaicine, piperine, allicin,[22] camphor and endocannabinoids,[23] the melastatin channel TRPM8 which responds to menthol and 1,8-cineole,[24] and the TRPA1 channel (highly expressed in human enterochromaffin cells) which responds to mustard oil, methyl salicylate, eugenol and cinnamaldheyde.[25]

After ingestion, these compounds act at esophageal level first, and then at gastric and duodenal level, stimulating gastric secretions with a general digestion-enhancing effect. Piperine increases pancreatic activity and reduces the intestinal transit time, while TRPA1 stimulation at duodenal level seems to mediate the release of CCK.[26]
Capsaicin activates TRPV1 receptors in the duodenum, which can also be activated and sensitized by acid. This activation stimulates gastrin secretion,[27] evokes dyspeptic symptoms (acutely, while if used chronically it reduces them) and affects gastric sensorimotor function.[28] At low dosages it stimulates the secretions of various gut peptides, in particular of calcitonin gene-related peptide (CGRP), which in turn stimulates microcirculation and protection of gastric mucosa from irritant compounds.[29]
The TRPA1 agonists cause 5-HT release in enterochromaffin cells and promote contraction of isolated strips of intestine via the 5-HT3 receptor. These results suggest that TRPA1 acts as a sensor molecule in enterochromaffin cells for the regulation of gastrointestinal functions. It stimulates vagal afferents and enteric nerves, which results in various gastrointestinal reactions, such as vomiting and peristaltic reflux.[30]
Allyl isothiocyanates, TRPA1 agonists, inhibit gastric ulcer formation in a similar but stronger fashion than the TRPV1 agonists (capsaicin and piperine) by modulating prostaglandin synthesis.[31]
Essential oils, and in general molecules capable of stimulating olfactory receptors, have been known for a long time to be able to produce physiological effects such as salivation, increasing appetite, etc. as part of the cephalic phase of motility and secretions of the gastrointestinal tract.[32]
It is therefore possible to envisage that a local stimulation of the tastant and olfactant receptors can act on afferent neurons (mainly of the vagus) with an effect on the CNS, on intrinsic efferent nerve fibers, with effect on the enteric nervous system, and on the rate of secretion of various peptides; which can then have effects on various systemic events, like mucosal proliferation, secretory processes, gastrointestinal motility, glucose homeostasis, etc.[33] This consequently affects functional disturbances of the gastrointestinal tract, like early satiety, sensation of fullness and meteorism, epigastric pain, abdominal cramps, nausea, motility disorders.
——————————————————————————————————————
[1] Kitamura, A., Torii, K., Uneyama, H., and Nijima, A. (2010) “Taste and health: Nutritional and physiological significance of taste substances in daily foods: Role played by afferent signals from olfactory, gustatory and gastrointestinal sensors in regulation of autonomic nerve activity”. Biol. Pharm. Bull, 33(11) 1778–1782; Dockray GJ. (2003) “Luminal sensing in the gut: an overview”. J Physiol Pharmacol.;54(Suppl 4):9–17; Rozengurt N, Wu S, Chen MC, et al. (2006) “Co-localization of the a-subunit of gustducin with PYY and GLP-1 in L cells of human colon”. Am J Physiol Gastrointest Liver Physiol.; 291:G792–G802
[2] Dockray 2003 Op. Cit.; Rozengurt. E. (2006) “Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut”. Am J Physiol Gastrointest Liver Physiol; 291(2):G171-7
[3] Wu, S. V., Rozengurt, N., Moon Yang, Young, S.H., Sinnett-Smith, J., and Rozengurt, E. (2002) “Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells”. PNAS, 99 (4):2392–2397
[4] Dockray 2003 Op. Cit.; Flemstrom, G. and Sjoblom, M. (2005) “Epithelial cells and their neighbors. II. New perspectives on efferent signaling between brain, neuroendocrine cells, and gut epithelial cells”. Am J Physiol Gastrointest Liver Physiol, 289: G377–G380
[5] Sternini, C. (2007) “Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing”. Am J Physiol Gastrointest Liver Physiol, 292: G457–G461
[6] Nozawa, K., Kawabata-Shoda, E., Doihara, H., Kojima, R., Okada, H., Mochizuki, S., Sano, Y., Inamura, K., Matsushime, H., Koizumi, T., Yokoyama, T., and Ito, H. (2009) “TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells” PNAS, 106(9):3408-3413
[7] Gollin L (2004) “Subtle and profound sensory attributes of medicinal plants among the Kenya Leppo`Ke of east Kalimatan, Borneo”. Journal of Ethnobiology, 24(2):173-201; Leonti M, Sticher O, Heinrich M (2002) “Medicinal plants of the Popoluca, México: organoleptic properties as indigenous selection criteria”. Journal of Ethnopharmacology, 81, 307-315; Pieroni A, Torry B (2007) “Does the taste matter?: taste and medicinal perceptions associated with five selected herbal drugs among three ethnic groups in West Yorkshire, Northern England”. Journal of Ethnobiology and Ethnomedicine, 3:21.
[8] Shepard GH (2004) “A sensory ecology of medicinal plant therapy in two Amazonian societies”. American Anthropologist, 106:2, 252-266.
[9] Scott K. (2005) “Taste recognition: food for thought”. Neuron, 48: 455– 464; Meyerhof, W., Behrens, M., Brockhoff, A., Bufe, B., & Kuhn, C. (2005) “Human bitter taste perception”. Chem senses; 30 Suppl 1(suppl 1), i14-5.
[10] Wu et al. 2002 Op. Cit.
[11] Behrens M, Meyerhof W (2010) “Oral and extraoral bitter taste receptors”. Results Probl Cell Differ.; 52:87-99
[12] Kidd, M., Modlin, I.M., Gustafsson, B.I., Drozdov, I., Hauso, O., and Pfragner, R. (2008) “Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants”. Am J Physiol Gastrointest Liver Physiol, 295: 260 –272
[13] Meyerhof, Behrens, Brockhoff, Bufe, & Kuhn, 2005 Op. Cit.
[14] Meyerhof, Behrens, Brockhoff, Bufe, & Kuhn, 2005 Op. Cit.
[15] Wicks D, Wright J, Rayment P, Spiller R. (2005) “Anticipatory physiological regulation in feeding biology: Cephalic phase responses” Eur J Gastroenterol Hepatol., 17(9):961-5
[16] Powers MA, Schiffman SS, Lawson DC, Pappas TN, Taylor IL. (1990) “The effect of taste on gastric and pancreatic responses in dogs.” Physiol Behav; 47(6):1295-7.
[17] Rozengurt 2006 Op. Cit.
[18] Wicks D, Wright J, Rayment P, Spiller R. (2005) “Impact of bitter taste on gastric motility”. Eur J Gastroenterol Hepatol;17(9):961-5
[19] Sternini 2007, Op. Cit.
[20] Jeon, T.-I., Zhu, B., Larson, J.L., and Osborne, T.F. (2008) “SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice”. The Journal of Clinical Investigation; 118(11):3693-3700
[21] Dotson CD, Zhang L, Xu H, Shin Y-K, Vigues S, et al. (2008) “Bitter Taste Receptors Influence Glucose Homeostasis”. PLoS ONE, 3(12): e3974
[22] Salazar H, Llorente I, Jara-Oseguera A, García-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T. (2008) “A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic”. Nat Neurosci;11(3):255-61
[23] Venkatachalam K, Montell C (2007) “TRP channels”. Annu. Rev. Biochem. 76: 387–417
[24] Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. (2004) “Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay”. Br. J. Pharmacol. 141 (4): 737–45
[25] Nilius B, Owsianik G, Voets T, Peters JA (2007) “Transient receptor potential cation channels in disease”. Physiol. Rev; 87 (1): 165–217
[26] Purhonen AK, Louhivuori LM, Kiehne K, Kerman KE, Herzig KH. (2008) “TRPA1 channel activation induces cholecystokinin release via extracellularcalcium”. FEBS Lett; 23;582(2):229-32
[27] Kidd M, Hauso Ø, Drozdov I, Gustafsson BI, Modlin IM. (2009) “Delineation of the chemomechanosensory regulation of gastrin secretion using pure rodent G cells”. Gastroenterology;137(1):231-41
[28] van Boxel OS, ter Linde JJ, Siersema PD, Smout AJ (2010) “Role of chemical stimulation of the duodenum in dyspeptic symptom generation”. Am J Gastroenterol;105(4):803-11.
[29] Abdel-Salam OM, Szolcsányi J, Mózsik G. (1997) “Capsaicin and the stomach. A review of experimental and clinical data”. J Physiol Paris; 91(3-5):151-71
[30] Nozawa, et al. 2009 Op. Cit.
[31] Matsuda H, Ochi M, Nagatomo A, Yoshikawa M. (2007) “Effects of allyl isothiocyanate from horseradish on several experimental gastric lesions in rats”. Eur J Pharmacol. 30;561(1-3):172-81
[32] Kitamura 2010 Op. Cit.
[33] Sternini, C. (2007) “Taste Receptors in the Gastrointestinal Tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing”. Am J Physiol Gastrointest Liver Physiol 292: G457–G461
