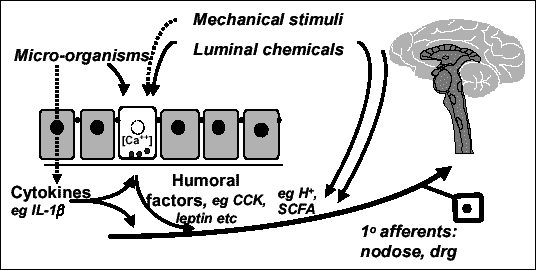
Nella terza installazione digestiva ho deciso di scardinare l’ordine cronologico della discussione saltando ad una serie di minimonografie di piante con un certo bagaglio di letteratura sperimentale e clinica, e che, più o meno tirate per i tricomi, possono dirsi piante anche alimentari. Ci sarà tempo per ritornare a discorsi di ordine più teorico.
——————————————————————————————-
Cynara cardunculus subsp. cardunculus Hayek — Asteraceae
Native to the Mediterranean area; the basal leaves were used as medicine as far back as ancient Roman times. It was used, as was characteristic of bitter remedies, primarily as a digestive and liver aid, to help stimulate the appetite, provide relief from nausea, stomach ache, flatulence, and a sense of fullness. More recently it has been described as liver protective, a choleretic, and a cholagogue. As with many members of the Asteraceae, it contains bitter-tasting sesquiterpene lactones, although the most characteristic and active compounds are caffeoylquinic acids (e.g. chlorogenic acid; 1,5-dicaffeoylquinic acid, neochlorogenic acid, criptochlorogenic acid, plus cinarine – 1,3-dicaffeoylquinic acid – only in hot acqueous extracts) and flavonoids.
Various experimental studies have shown promising choleretic and digestive activities of the dry extract of artichoke leaves. Total extract, fenolic acids and caffeoylquinic acids all show choleretic effects both in vitro1 and in vivo2 and they increases gastrointestinal peristalsis.3
These experimental studies are partly supported by clinical investigations that have shown that artichoke preparations may relieve digestive complaints (sensation of fullness, loss of appetite, nausea and abdominal pain) through an increase in the formation and flow of bile, probably mainly due to the presence of flavonoids and caffeoylquinic acids, but also via the bitter-tasting sesquiterpene lactones.4 In fact, the German Commission E approves the use of fresh or dried artichoke leaf for dyspeptic problems due to its choleretic action,5 and the ESCOP monograph reports as indications digestive disorders such as stomachache, nausea, emesis, sensation of fullness, flatulence.6
A post-marketing surveillance study reported by Held7 included 417 patients with hepatic and biliary tract disease treated for four weeks with artichoke leaf extract (1% caffeoylquinic acids, 1500 mg/day). In 65-77 % of patients, abdominal pain, bloating, meteorism, constipation, lack of appetite, and nausea were eliminated after one week, and in 52-82% of patients, after four weeks.
A mode of action study (a crossover, randomized, double blind clinical study, vs. placebo, with one-day treatment periods separated by an eight-day washout period) using an aqueous dry extract (4.5-5:1) on 20 subjects with acute or chronic metabolic disorders, showed that administration of six capsules (total weight 1.92 g) intraduodenally caused a peak increase (100 to 150 % compared to baseline) in bile one hour later, which lasted for three hours. The study inferred, but did not demonstrate, therapeutic benefit for dyspepsia. It suffered from many shortcomings: the study was too short, the sample size was small, it wasn’t conducted on subjects with dyspepsia, and the product was not delivered orally.8
A second post-marketing surveillance study included 553 subjects with dyspepsia, who were administered an average of 1.5 g of dry extract (3.8-5.5:1) for an average of 43.5 days. There was a clinically relevant reduction in dyspeptic symptoms for 71% of the subjects in 6 weeks of treatment (66% flatulence, 76% abdominal pain, 82% nausea and 88% emesis).9
Walker, Middleton, and Petrowicz reported an analysis of a patient subset from the initial survey by Fintelmann and Menssen with key symptoms of irritable bowel syndrome (n=279). These patients experienced significant reductions in symptoms (emesis 95%, nausea 85%, abdominal pain 75%).10
In a similar open study that lasted six months on 203 subjects suffering from dyspepsia, there was an average reduction (after 21 days) of 66% of the same range of symptoms. The global efficacy was evaluated by the physicians as being good or excellent in 85.7% of the cases.11
In a more recent double-blind, randomized controlled trial vs. placebo on 244 patients with
functional dyspepsia, the verum treatment (1920 mg dry extract/die) improved/reduced? symptoms and improved quality-of-life measures after six weeks.12
In an open, dose-ranging postal study, 454 healthy patients with self-reported dyspepsia were treated with a dry extract, at 320 or 640 mg daily. Both dosages reduced all dyspeptic symptoms, with an average reduction of 40% in global dyspepsia score.13 A subset analysis of the study, identifying post hoc 208 subjects suffering with IBS, showed a significant fall in disease incidence of 26.4% after 2 months.14
Thirty subjects with functional dyspepsia consumed an iced dessert with or without artichoke extract. The results show that ingestion of the dessert induces a reduction of symptom severity and range even without the extract, but that the presence of the extract intensifies the effects.15
A prospective cohort study on 311 patients with functional dyspepsia analyzed the efficacy of a commercial mixture of dry extracts of artichoke leaf (15% of chlorogenic acid – 150 mg per capsule), dandelion radix (Taraxacum officinalis – 2% of inulin), turmeric rhizome (Curcuma longa – 95% of curcumin) and rosemary (Rosmarinus officinalis) microencapsulated essential oil. After 60 days of treatment, a statistically significant gradual reduction in symptom severity was noted, and a global clinical response, defined as a 50% reduction in the total scores of all symptoms, was recorded in 38% of patients at 30 days.16
Taraxacum officinale G.H. Weber ex F. H. Wigg — Asteraceae
A perennial weed widely distributed in the warmer temperate zones of the Northern Hemisphere. Its roots and rhizomes have been used extensively since ancient times in Europe as a bitter tonic and for the treatment of various disorders such as dyspepsia, heartburn, spleen and liver complaints, hepatitis and anorexia.17
The intensely bitter-tasting compounds are sesquiterpene lactones of the eudesmanolide, guaianolide and germacranolide types18 (previously known as taraxacin) and are unique to Taraxacum. The drug contains also triterpene alcohols (taraxastane-type), phytosterols , lupane-type triterpene and hydroxycinnamic acids.19
In Germany, Commission E supports using Taraxacum officinale folia to treat loss of appetite, dyspepsia, bloating and flatulence, while radix with folia is recommended to treat disturbed bile flow, loss of appetite and dyspepsia.20
ESCOP recommends T. officinale radix for restoring hepatic and biliary function, dyspepsia and loss of appetite.21
Intragastric administration of an aqueous or 95% ethanol extract of the whole plant (dose non specified) to rats increased bile secretion by 40% in over 2 hours.22
Dandelion decoction administered by injection to dogs doubled the volume of bile secreted.23 A similar effect was observed following intraduodenal administration to rats.24
An herbal combination containing Calendula officinalis, Taraxacum officinale, Hypericum perforatum, Melissa officinale and Foeniculum vulgare reduced intestinal pain in 96% of 24 patients by the 15th day in an uncontrolled trial involving patients with chronic colitis. Defecation was normalized in patients with diarrhea syndrome.25
A prospective cohort study on 311 patients with functional dyspepsia analyzed the efficacy of a mixture of dry extracts of artichoke leaf, dandelion radix, turmeric rhizome and rosemary essential oil. After 60 days of treatment, a statistically significant gradual reduction in symptom severity was noted; and a global clinical response – defined as a 50% reduction in the total scores of all symptoms – was recorded in 38% of patients at 30 days.26
Silybum marianum (L.) Gaertn. — Asteraceae
Plant indigenous to North Africa, Asia Minor and southern Europe, have been used since ancient times for the treatment of gastrointestinal and hepatic complaints like anorexia,27 colic and abdominal cramps,28 and nausea.29
It is nowadays mainly known for its important hepatoprotective and hepartoregenerative activity but, being a bitter remedy, it shows classic digestive activity, although the clinical evidence is lacking (Commission E recognizes this by suggesting its use as a treatment of dyspeptic complaints). The seeds contain a complex mixture of flavolignans termed silymarin, which seems to be the main active principle ingredient?.30 Other important compounds are the flavonoids quercetin, dehydroksampferol and (+)-taxifolin, saponins, poliacetylenes and essential oils.
An experimental study has shown that intragastric administration of an acetone extract of fruit containing silybin increased the volume and dry mass of excreted bile in rats.31
Although there are no direct clinical data, Silybum marianum has been tested in a fixed preparation combining Iberis amara, Melissa officinalis, Matricaria recutita, Carum carvi, Mentha x piperita, Glycyrrhiza glabra, Angelica archangelica, Silybum marianum and Chelidonium majus. This preparation has shown a general activity on dyspepsia,32 in particular: a reduced acid output, increased mucin secretion, prostaglandin E(2) release and a decrease in leukotriene levels.33 A second study showed protection against the development of gastric acidity rebound/reflux? and inhibited the serum gastrin level in rats.34
Citrus limon (L.) Burmann fil. — Rutaceae
The rind has been used both in the West and in the East as a tonic digestive, strongly aromatic and slightly bitter, used in decoction and alcoholic extracts. In Traditional Chinese Medicine it is used to resolve gastrointestinal disorders such as abdominal and epigastric bloating, belching, nausea and emesis, inappetence and diarrhea, and in the West for colicky indigestions, abdominal bloating, slow digestion, and nervous dyspepsia. The juice has traditionally been used as a digestive, astringent, stomachic, antispasmodic and carminative, used for the treatment of inappetence, gastralgy, nausea, and gastric acid reflux.35
The epicarp is very rich in an essential oil dominated by monoterpenes (60-95%) in particular R(+)-limonene (up to 70%), and linear aldheides (citrals), but also characterized by photoactive furocoumarins (more than 5%).36 Also present are phenolic compounds, flavonoids, pectin and various organic acids from the juice.37
Lemon juice and extracts exert both indirect and direct actions on gastrointestinal activity. Particularly interesting are the indirect (cognitive and sensory mediated) ones.
It is well known that both lemon aroma and flavor are sialagogues, that is, that they influence the cephalic phase of digestion (salivation). In an interesting study comparing the effects of different visual stimuli, Christensen and Navazech show that there is a stronger response (a higher salivary volume) to visual stimuli of acidic (lemon juice) and pungent foods (pizza with hot peppers).38 Lemon odor and the introduction of lemon juice into the oral cavity both cause an increase in the volume of saliva, statistically higher than that caused by a non-stimulus (pure air or pure water).39 Moreover, according to Davenport, the lemon juice seems to be able to influence not only the quantity but also the quality (in terms of compositions) of the saliva, which becomes richer in proteins.40 This suggests that prior knowledge of the food items can modify the cephalic phase of digestion, and reinforces the thesis of a relationship between secondary metabolites and gastrointestinal physiology: the presence or the supposed presence of possibly hazardous substances (irritant, bitter, astringents, etc.) not only alters the salivary volume but increases the percentage of compounds like praline, which offer a certain degree of protection from irritant compounds such as tannins.
The study by Bauslaugh showed a relationship between salivation and gastrointestinal motility during olfactory stimulation,41 and it is well known that pepsinogen, gastrin and HCl secretions are influenced by cephalic stimulations,42 hence possibly by the organoleptic stimulation by lemon juice.
The palatability and the absence of gastrointestional adverse symptoms have already been tested in a commercial product containing lemon juice and various herb extracts.43
Apart from sensory, indirect activities, lemon juice can directly affect gastrointestinal secretions. 100 ml. of orange and lemon juice have shown very potent stimulant action on pancreatic secretion (higher output, higher bicarbonate content, higher enzymatic content), compared to other stimuli, and the peak response was observed earlier (60 minutes for the juice, 90 minutes for the other stimuli). In general the juice had a response quantitatively and qualitatively comparable to secretin.44
The fruit juice and some of its components (caffeic acid, ferulic acid, hesperidine, p-coumaric acid) show choleretic/cholagogue and antispasmodic action.45
The essential oil and many of its components are rubefacient46 and stimulant carminative;47 limonene is myorelaxant and antispasmodic at high doses, as are many of the other essential oil components: a-pinene, a-terpinene, bergapten, b-pinene, cariophillen, geraniol, mircene, nerale, terpinen-4-olo.48 Hence, they may? could play a role in the antispasmodic and carminative activity of lemon rind extract and lemon juice.
Pectin shows stomachic action and gastrointestinal protective activity.49
Foeniculum vulgare Mill. — Apiaceae
Commonly employed as a culinary herb and as a remedy to improve digestion in traditional systems of medicine; they have been used since ancient Roman and Egyptian times as a valuable warming carminative and aromatic digestive. Fennel has? also had a persistent/consistent reputation as an ingredient in “gripe water” and other remedies for infant colic.
Given in the form of a homemade tea or infusion, fennel is a useful standby for dyspepsia, bloating and flatulence, and poor appetite.50
It contains an essential oil mainly composed of trans-anethole (30-90%), (+)-fenchone (6-30%) and estragole (methylchavicol – ca. 5% ); and also contains flavonoids and coumarines.
Only a handful of modern clinical trials are reported in the literature, demonstrating – together with its widespread traditional use – a reduction in colic in babies, and a reduction in the symptoms of chronic colitis.
A randomized placebo-controlled trial tested a fennel seed oil emulsion, compared with placebo, on 125 infants, 2 to 12 weeks of age, diagnosed with colic. The emulsion eliminated colic in 65% of infants in the treatment group, compared to 23.7% of infants in the control group with an Absolute Risk Reduction (ARR) = 41%.51
A mixture containing chamomile (Matricaria recutita), fennel (Foeniculum vulgare) and lemon balm (Melissa officinalis) was found to have significant benefits in the treatment of infantile colic in a double-blind, placebo-controlled study on 93 breastfed infants, treated twice a day for 1 week. Crying time reduction was observed in 85.4% of subjects.52
However, two subsequent experimental studies in mice, while confirming the reduction of intestinal motility at dosages similar to those used in human trials, showed that the major contribution to the antispasmodic activity is due to Matricaria recutita and Melissa officinalis.53
In an uncontrolled clinical study twenty-four patients with chronic non-specific colitis were treated with a herb combination of Taraxacum officinale, Hipericum perforatum, Melissa officinaliss, Calendula officinalis and Foeniculum vulgare. As a result of the treatment, the spontaneous and palpable pains along the large intestine disappeared in 95.83 per cent of the patients by the 15th day of their admission to the clinic.54
Backed by weaker clinical evidence, but supported by very widespread and validated traditional use, are the indications for digestive complaints: dyspepsia with bloating and flatulence, poor appetite and indigestion.55
In animal studies, fennel has been shown to be antispasmodic, prokinetic and secretagogue.
Isolated trans-anethole reduced contractions of a rat diaphragm preparation;56 the essential oil seems able to reduce smooth muscle spasms in various in vitro models,57 but this activity seems concentration -dependent, with spasmogenic effect at lower doses, and spasmolytic at higher ones.58
The ethanol extract59 and the aqueous infusion show60 spasmolytic effects.
Intragastric administration of 24.0 mg/kg bw of the fruits increased spontaneous gastric motility in unanaesthetized rabbits.61
An aqueous extract of the fruits (10% p/v), administered to anaesthetized rats via gastric perfusion at 0.15 ml/minutes, significantly increased gastric acid secretions.62
The admixture of 0.5% fennel fruits to the diet of rats for 6 weeks reduced the food transit time by 12%,63 while the admixture of fennel fruits (0.5%) and mint (1%) for 8 weeks stimulated a higher rate of secretion of bile acids in rats, and a significant enhancement of secreted intestinal enzymes, particularly lipase and amylase.64
Melissa officinalis L. — Lamiaceae
A very popular traditional herb used in infusion for restlessness and dyspepsia, especially among children. It contains very low amounts of essential oil (0.02-0.37%), organoleptically characterized by the aldheydes geranial and neral, and 6% of rosmarinic acid.
There is a dearth of clinical research on the digestive activity of Lemon balm, and the only two existing clinical studies are based on formulas and not on the single herb extract.
A randomized, double-blind, placebo-controlled trial showed significant improvement of colic in babies given a chamomile, fennel and lemon balm preparation compared with babies given a placebo.65
A fixed commercial combination of extracts of Melissa officinalis, Mentha spicata and Coriandrum sativum was tested on 32 irritable bowel syndrome patients and compared to placebo for 8 weeks in a clinical study. The study shows that the combination reduces the severity and frequency of abdominal pain and of bloating better than placebo.66
There is little doubt that the ethanol fraction of the plant, and in particular the volatile oil component, is responsible for the antispasmodic activity of the herb. The ethanol extracts and the essential oil have shown inhibition of artificially-induced contraction of smooth muscles,67 but there are also contrasting data.68 The essential oil was more effective than its isolated components, but it has been observed that neral and geranial were more active than citronellal and β-cariophillen.69
Mentha xpiperita L. — Lamiaceae
Without doubt one of the most world-renowned aromatic plants, and the most important one in terms of annual production, both of the dried herb and essential oil. It has always been used in traditional learned and folk medicine as a carminative, antispasmodic, antiemetic and digestive, both in the West and in the East.
It contains a large amount of essential oil characterized by the presence of a monoterpene alcohol, menthol, which is responsible for many of the activities of the herb and for its characteristic aroma. It also contains flavonoid compounds and hydroxycinnamic compounds.70
Peppermint has also been the focus of a fair amount of good quality research, both experimental and clinical, that supports many of the traditional claims. In particular it has shown antispasmodic, carminative and choleretic activities.
The essential oil reduces intracolonic pressure.71 In an open study of 20 patients, peppermint essential oil used alongside a colonoscope relieved colonic spasms,72 and it had the same effect when administered with barium enemas.73
The essential oil is also able to reduce tension in hypertonic intestinal smooth muscles? in cases? of IBS.74
In healthy volunteers, intragastric administration of a dose equivalent to 180 mg peppermint oil, reduced intraesophageal pressure within 1-7 minutes of infusion.75
Oral administration of the essential oil delayed the gastric emptying time in healthy volunteers and in patients with dyspepsia,76 and it slowed small intestinal transit time in 12 healthy volunteers.77
A combination of essential oils (90 mg of peppermint and 50 mg of caraway in an enteric-coated capsule) was tested in various studies.
The combination produced smooth muscle relaxation of stomach and duodenum;78 in a double-blind, placebo-controlled multicentre trial with 45 patients it improved symptoms of dyspepsia, reducing pain in 89.5% of patients and improving Clinical Global Impression scores in 94.5% of patients.79
The same combination tested on 223 dyspeptic patients in a prospective, randomized, reference and double-blind controlled multicentre trial, significantly reduced pain,80 and tested on 96 outpatients with dyspepsia, significantly reduced pain by 40% and reduced sensations of pressure, heaviness and fullness.81
The formula was shown to be as effective as Cisapride in reducing both the magnitude and frequency of pain,82 and it had a relaxing effect on the gall bladder.83
In a systematic review of herbal medicines for functional dyspepsia, the authors found 17 randomized clinical trials, nine of which involved peppermint and caraway combination preparations. Symptoms were reduced by all treatments; 60- 95% of patients reported improvements in symptoms.84
The essential oil and fractions of it have been shown to have gastrointestinal smooth muscle relaxant activity and to inhibit spontaneous smooth muscle contractions both in vitro and in vivo.85 Some data are also available on the antispasmodic effects of the ethanol extract, as well as flavonoids isolated from the leaf.86
The carminative activity of peppermint is, in fact, probably due to a relaxing action on the gastrointestinal sphincters,87 and to a reduction of the volume of intestinal gas by the antimicrobial, anti-fermentative and antifoaming effects of the essential oil.88
Choleretic activity (increased bile secretion and increased synthesis of bile acids) has been demonstrated in animal models for the herb, various flavonoid fractions, flavomentin, the essential oil and menthol.89 The effect probably derives from the spasmolytic activity of menthol and other terpenes on the Oddi’s sphincter.90
The essential oil seems to be acting by interacting with smooth muscle Ca channels probably by inhibiting the influx of extracellular Ca ions without effects on their intracellular mobilization, and menthol has been isolated as the most important compound.91
The antiemetic and prokinetic effects of peppermint oil and of (-)-menthol are due at least partly to their binding to the 5-HT(3) receptor ion-channel complex, in a manner similar to that of ginger.92
Matricaria chamomilla L. — Asteraceae
It has been a highly popular family herb since antiquity, generally used for nervous excitability and digestive disorders, stomach cramping, dyspepsia and flatulence. This tradition of use notwithstanding, much of the research data concentrate on antinflammatory and vulnerary activity.
However, its antispasmodic and relaxant effects provide a theoretical basis for its use in gastrointestinal conditions, and the German Commission E approves chamomile for gastrointestinal spasms and inflammatory diseases of the gastrointestinal tract.
It contains an essential oil, flavonoids (in particular apigenin and its related flavonoid glycosides), and proazulenes (sesquiterpene lactones) including matricin, matricarin and desacetlymatricarin.93
Although a well-known and widely used herb, almost no substantial clinical research exists into the remedy’s use for digestive disorders, and only a few well designed clinical studies are available There are however some experimental studies pointing to antispasmodic activity of the plant and its constituents on smooth muscle.
In an open, multicentre study, 104 patients with gastrointestinal complaints (gastritis, flatulence and mild intestinal spasms) were treated for 6 weeks with an oral chamomile extract (standardized for 0.05% alpha-bisabolol and 0.15% apigenin-7-glucoside), with 44.2% of subjects self-reporting symptom free.94
In a double-blind study, a herbal decoction (150 mL/day containing Matricaria chamomilla, Verbena officinalis, Glycyrrhiza glabra, Foeniculum vulgare and Melissa officinalis) was tested for seven days on 68 healthy infants with colic. 57% of the infants experienced relief compared to 26% in the placebo group.95
In another trial – prospective, double-blind and randomized – a preparation containing chamomile extract and pectin was tested on children aged 6 months to 5.5 years with uncomplicated diarrhea. The preparation reduced duration and severity of diarrhea significantly faster than the placebo.96
Whole extracts and isolated components demonstrate a dose-dependent antispasmodic effect in vitro.97 The major activity was related to (-)-α-bisabolol, the cis-spiroethers,98 and the flavonoids (in particular apigenin).99 Bisabolol oxides A and B, the essential oil, other flavonoids and the small amount of coumarins were less active in vitro.100
The mechanisms for chamomile antispasmodic activity are still unclear. However, at least one mechanism has been proposed: the inhibition of cAMP- and cGMP-phosphodiesterases by flavonoids.101
Chamomile also increases production of bile by the liver.102
Cymbopogon citratus (DC.) Stapf. — Poaceae
An aromatic tropical grass, whose essential oil is characterized by the presence of citrals (up to 90%) and monoterpenes. It also contains triterpenoids and flavonoids. It has been traditionally deemed a carminative, a light sedative, an analgesic, an antiemetic and an antispasmodic.103
It is most frequently used as a remedy for gastrointestinal disorders, in particular stomachache, acids indigestion, abdominal cramps, diarrhea, and dyspepsia.104
There are, however, no clinical data available, and only a few experimental data showing that di essential oil is strongly antispasmodic and carminative.105
Aloysia citrodora Palau — Verbenaceae
Used in Latin America, USA and Europe as an aromatic ingredient in fruit salads, jams, cold drinks or as an infusion.106 The plant is rich in an essential oil dominated by citrals. It also contains flavonoids, phenolic acids and tannins.107
Various authors report its digestive,108 spasmolytic,109 stomachic,110 and carminative activity,111 and it has been used for gastrointestinal and spasmodic disorders, e.g. flatulence, dyspepsia, colic, nausea, etc.112
There are, however, no clinical data in support of these claims, but there are some experimental data.
The essential oil and many of its components are aperitive, analgesic/antinociceptive, and antispasmodic.113 . Cineole and borneol are choleretics and secretagogues.114
Chlorogenic acids could act synergistically with the essential oil as digestives, and vitexin shows an antispasmodic activity.115
Caceres reports of a clinical study (impossible to track down) which allegedly shows a positive effect of the plant on inappetence, slow digestion, gastralgy and emesis.116
Illicium verum, Hook. f. — Illiciaceae and Pimpinella anisum L. — Apiaceae
Both popular aromatic remedies: the first in China, where it is a component of the very famous five spice powder, together with cinnamon/cassia, cloves, fennel and Sichuan pepper; and the second in Europe and North America. Both are used quite often in food recipes (Star Anise in garam masala spice blends and the rice dish biriyani, and Aniseed in many flavored drinks)
These two fruits are deemed carminative and stomachic and they are taken internally in the treatment of abdominal pain and digestive disturbances. They are often included in remedies for indigestion, and they are believed to be effective for children’s digestive upsets, including colic pain.117 Some people chew the fruits after meals for better digestion. The essential oils are used as a stimulant, stomachic, carminative.
Star Anise is characterized by its essential oil content, particularly rich in prenylated C6-C3 compounds (phenylpropanoids: anethole and its analogues, estragole, eugenol), but contains also neolignans and sesquiterpenes in addition to several common flavonoids, diterpenoids and triterpenoids.118
Aniseed contains an essential oil whose major component is trans-anethole.
Their traditional use notwithstanding, there is no clinical evidence supporting it, but there are limited experimental data. The essential oil of Aniseed and trans-anethole both seem to act as antispasmodics in vitro and in vivo on animal models. They antagonize artificially-induced spasms and decrease the rate and extent of contractions of smooth muscle preparations,119 possibly via Ca-channel blockage and the NO-cGMP pathway.120 Many authors point to antispasmodic,121 carminative122 and digestive activities,123 suggesting use for abdominal colic, inappetence, dyspepsia, flatulence.
Zingiber officinale Roscoe — Zingiberaceae
Probably one of the oldest domesticated spices in human history. It has a prominent role in Asian systems of medicine where it is used for the treatment of dyspepsia, flatulence, colic, vomiting, diarrhea, spasms and to stimulate the appetite, but over the centuries has become part of western cuisine and pharmacopoea as well.124
The ginger rhizome contains an essential oil (1-4%) and a resin, known collectively as oleoresin. The chief constituents of the essential oil are the sesquiterpenes a-, and b-zingiberene, which are responsible for the characteristic aroma. The resin contains pungent phenolic compounds called gingerols, gingerdioles and gingerdiones, and their corresponding dehydration products known as shogaols.125
According to the studies, ginger exerts several effects in the gastrointestinal tract: secretagogue (saliva, bile, pancreatic juices, gastric juices), antiemetic, intestinal antispasmodic and gastric prokinetic.
It stimulates the flow of saliva, bile and gastric secretions.126 An extract containing the oleoresin and administered intraduodenally to rats produced an increase in the bile secretion, while the aqueous extract was not active. These results point towards the oleoresin as the active principle, and in fact it was shown that [6]- gingerol and [10]-gingerol were mainly responsible for the cholagogic effect.127 An oral dose of ginger enhanced rat pancreatic lipase, sucrase and maltase activity, and stimulated trypsin and chymotrypsin.128
The essential oil,129 a 95% ethanol extract,130 a hot water extract131 and of a formula containing ginger, Pinellia ternata, Citrus aurantium, Pachyma hoelen, and Glycyrrhiza glabra,132 were all shown to possess antispasmodic activity on intestinal smooth muscles.
The rhizome extract, shogaols and gingerols all increased gastric motility in animal models and in humans.
In a clinical study ginger, assumed before meals, increased number and frequency of contractions in the corpus and in the antrum, and frequency of contractions in the duodenum. Assumed after meals, it contributed to motility to a lesser degree.133
Ginger and a Japanese formula (Dai-Kenchu-To) containing ginger, Zanthoxylum fruit and ginseng root, both induced phasic contractions in the gastric antrum..134
Previous clinical data had shown that ginger did not affect the gastric emptying rate.135 There were suggestions, however, that this lack of activity was due to low dosage of ginger rhizome.
The prokinetic activity was confirmed in other in vitro and in vivo tests, that showed that ginger extract enhances the intestinal transit of charcoal meal and that it acts through a spasmogenic, dose-dependant cholinergic agonistic effect on the post-synaptic muscarinic M3 receptors in the stomach fundus. It also has a possible inhibitory effect on pre-synaptic muscarinic autoreceptors. At the same time it shows spasmolytic activity at the intestinal level (also possessed by 6-shogaol, 6-gingerol, 8-gingerol and 10-gingerol), probably through a Ca2 + antagonist effect.136
Various constituents found in Ginger, 6-, 8- and 10-gingerol, 6-shogaol, and galanolactone, act as serotonin receptor antagonists, which could explains the antispasmodic effects on visceral smooth muscle.137 They could exert their effect by binding to receptors in the signal cascade behind the 5-HT(3) receptor ion-channel complex, perhaps substance P receptors or muscarinic receptors.138
At the same time two compounds (10-shogaol and 1-dehydro-6-gingerdione), and particularly the whole lipophilic extract, have shown to partially activate the 5-HT(1A) receptor (20-60% of maximal activation).139
The serotonin receptor antagonist activity may partly explain the antiemetic effect of ginger, since these receptors do mediate peristalsis and emesis,140 and the constituents active on these receptors were also active as anticholinergic antiemetics, in the following descending order of potency: 6-shogaol> or= 8-gingerol>10-gingerol> or = 6-gingerol.141
Many clinical studies have shown the positive antiemetic effects (prevention and treatment of nausea) of Ginger and many of its constituents (shogaols, gingerols, zingerones) under different circumstances.142
A systematic review of six controlled studies found that Ginger was more effective than placebo in some studies of postoperative nausea and vomiting. Of the three studies conducted in postoperative nausea and vomiting, two suggested that Ginger was superior to placebo and equally as effective as metoclopramide, whereas one found no benefit.143
A recent Cochrane review on 20 trials concluded that Ginger might be of benefit in cases of nausea and emesis, but that the evidence to date was weak.144
Capsicum annuum L. — Solanaceae
A native American plant that has been exported all over the world and has conquered both the cuisine and the medicine of Europe, Africa and Asia, in a very interesting reverse spice journey.
Capsicum’s main active chemical group is that of the capsaicinoids, a group of pungent alkaloids whose prototype is capsaicin (8-methyl-N-vanillyl-6-nonenamide), which has been tested for its analgesic effect.145
The scientific evidence about capsicum and capsaicinoids and their effects on the gastrointestinal tract is rather contrasting. It is well known that capsaicin can interact with the vanilloid receptor VR1 and that this interaction can lead to direct and indirect effects. The interaction causes a selective impairment of the activity of nociceptive C-type fibers carrying pain sensations to the central nervous system, causing, on chronic dosage, analgesic and anti-inflammatory effects.146 These have been evaluated in patients suffering from heartburn147 and functional dyspepsia, with encouraging results.148
The data on gastric secretions and motility are less clear: some studies found a stimulation of gastric emptying149 and of secretions,150 others found no difference,151 and others even found a reduction in activity.152
The intake of red pepper has caused a reduced energy intake, suppression of hunger and increased satiety,153 an activity in line with a possible effect on the secretion of CCK.
Carica papaya L. — Caricaceae
A tropical plant original to the dry flatlands of Mesoamerica (South Mexico and Guatemala). The fruit is one of the most important fruits in the tropics and worldwide, and its fermented products are well known in the field of FF for its antioxidant properties.154 Its juice and jams made out of the fruit pulp are used also for digestive complaints (constipation, diarrhea, dyspepsia, enteritis), and it is supposed to possess carminative, cholagogue and digestive activities.155 It contains various proteolytic enzymes like papaine and chymopapaine; carotenoids, monoterpenoids; and organic acids.156
Papaine is a proteasis (similar to bromelin) with wide-range specificity, it hydrolyses polypeptides, amides and esters, particularly when used in an alkaline environment, and is used in digestive disorders; chymopapaine is very similar but less active. Although the mature fruit is less rich in papaine than the unripe one, the quantity is still sufficient to give a biological bases to the traditional digestive claims for the fruit and its derivatives.
——————————————————————————————-
Note
1 ESCOP Monographs on the medicinal uses of plant drugs. Devon, European Scientific Cooperative on Phytotherapy, 2003
2 Preziosi P, Loscalzo B “L’azione sulla coleresi, sul colesterolo ematico e sulla lipoidosi colesterolica del principio attivo del carciofo e di sostanze ad esso correlate”. Fitoterapia. 1956; 27:666-72; Bombardelli E, Gabetta B, Martinelli EM, “Gas-liquid chromatographic and mass-spectometric investigation of Cynara scolymus extracts”. Fitoterapia. 1977; 48:143-152.
3 Lietti A. “Choleretic and cholesterol lowering properties of two artichoke extracts”. Fitoterapia. 1977; 48:153-58
4 Kraft K “Artichoke leaf extract–Recent findings reflecting effects on lipid metabolism, liver and gastrointestinal tracts”. Phytomedicine. 1997; 4 (4): 369-378; Wegener T, Fintelmann V. “Pharmacological properties and therapeutic profile of artichoke (Cynara scolymus L.)”. Wien Med Wochenschr. 1999;149(8-10):241-7.
5 Blumenthal M, Busse W, Hall T, Goldberg A, Gruenwald J, Riggins C, Rister S, eds. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Trans. S Klein. Austin, TX: American Botanical Council, 1998
6 ESCOP 2003 Op. Cit.
7 Held C “Artischoke bei Gallenwegsdyskinesien: Workshop “Neue Aspekte zur Therapie mit Choleretika.” Kluvensiek. 1991; 2: 9. Cited in Kraft 1997 Op. Cit.
8 Kirchhoff R, Beckers CH, Kirchhoff GM, Trinczek-Gartner H, Petrowicz O, Reimann HJ “Increase in choleresis by means of artichoke extract”. Phytomedicine. 1994; 1: 107-115
9 Fintelmann V. “Antidyspeptische und lipidsenkende Wirkungen von Artischockenextrakt: Ergenbnisse klinischer Untersuchungen zur Wirksamkeit und VertraNglichkeit von Hepar-SL forte an 553 Patienten”. Zeitschrift fur Allgemeinmedizin. 1997; 72 (Suppl. 2): 3-19. Cited in Kraft 1997 Op. Cit.; Fintelmann V, Menssen HG. “Aktuelle Erkenntnisse zur Wirkung von ArtischockenblaNtterextrakt als Lipidsenker und Antidyspeptikum”. Deutsche Apotheker-Zeitung. 1996; 136: 1405. Cited in Kraft 1997 Op. Cit.;
10 Walker AF, Middleton RW, Petrowicz O “Artichoke leaf extract reducessymptoms of irritable bowel syndrome in a post-marketing surveillance study”. Phytotherapy Research. 2001; 15 (1): 58-61
11 Fintelmann V, Petrowicz O Naturamed. 1998; 13:17-26
12 Holtmann G, Adam B, Haag S, Collet W, Grunewald E, Windeck T. “Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: a six-week placebo-controlled, double-blind, multicentre trial”. Aliment Pharmacol Ther. 2003 Dec;18(11-12):1099-105
13 Marakis G, Walker AF, Middleton RW, Booth JC, Wright J, Pike DJ. “Artichoke leaf extract reduces mild dyspepsia in an open study”. Phytomedicine. 2002 Dec;9(8):694-9.
14 Bundy R, Walker AF, Middleton RW, Marakis G, Booth JC. “Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: a subset analysis” J Altern Complement Med. 2004; Aug;10(4):667-9)
15 Gasbarrini G, Zaccone V, Covino M, Gallo A. “Effectiveness of a “cold dessert”, with or without the addition of a mixture of digestive herbs, in subjects with “functional dyspepsia” J Biol Regul Homeost Agents. 2010; Jan-Mar;24(1):93-8
16 Sannia A. “Phytotherapy with a mixture of dry extracts with hepato-protective effects containing artichoke leaves in the management of functional dyspepsia symptoms”. Minerva Gastroenterol Dietol. 2010 Jun;56(2):93-9
17 Schutz K, Carle R, Schieber A. “Taraxacum–a review on its phytochemical and pharmacological profile”. J Ethnopharmacol. 2006; Oct 11;107(3):313-23; Guarrera PM. “Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium)”. Fitoterapia. 2005; Jan;76(1):1-25
18 Hansel R, Kartarahardja M, Huang JT, Bohlmann F. “Sesquiterpenlacton-beta- D-glucopyranoside sowie ein neues Eudesmanolid aus Taraxacum officinale“. Phytochemistry. 1980; 19:857-861; Zielinska, K. and W. Kisiel. “Sesquiterpenoids from roots of Taraxacum laevigatum and Taraxacum disseminatum“. Phytochemistry. 2000; 54: 791-794
19 Hansel et al. 1980 Op. Cit.; Rauwald H-W, Huang JT. (1985) “Taraxacoside, a type of acylated gamma-butyrolactone glycoside from Taraxacum officinale“. Phytochemistry. 1985; 24:1557-1559; Williams CA Goldstone F Greenham J “Flavonoids, cinnamic acids and coumarins from the different tissues andmedicinal preparations of Taraxacum officinale“. Phytochemistry. 1996; 42(1):121-7
20 Blumenthal et al 1998 Op. Cit.
21 ESCOP 2003 Op. Cit.
22 Bohm K “Studies on the choleretic action of some medicinal plants” Arzneimittelforschang. 1959; 9:376-378
23 ESCOP 2003 Op. Cit.
24 ESCOP 2003 Op. Cit.
25 Chakurski I, Matev M, Koichev A, Angelova I, Stefanov G. “Treatment of chronic colitis with an herbal combination of Taraxacum officinale, Hypericum perforatum, Melissa officinalis, Calendula officinalis and Foeniculum vulgare“. Vutr Boles. 1981; 20:51-54.
26 Sannia A. 2010 Op. Cit.
27 Fleming, T. et al. PDR for Herbal Medicine, 1st ed., Medical Economics Co., Montvale, NJ, 1998
28 Blaschek W et al., eds. Hagers Handbuch der pharmazeutischen Praxis. Folgeband 2: Drogen A-K, 5th ed. Berlin, Springer-Verlag, 1998
29 Mills, Bone, 2000 Op. Cit.; Morazzoni P, Bombardelli E. “Silybum marianum (Carduus marianus)”. Fitoterapia. 1995; 66:3-42. , Blumenthal et al. 1998 Op. Cit.; WHO WHO Monographs on Selected Medicinal Plants. 2 vol. Geneva: World Health Organization; 2002, 300-316
30 Saller R, Meier R, Brignoli R. “The use of silymarin in the treatment of liver diseases”. Drugs. 2001; 61:2035-2063)
31 Danielak R, Popowska E, Borkowski B. “The preparation of vegetable products containing isofraxidin, silibin, and Glauciumalkaloids and evaluation of their choleretic action”. Polish Pharmacology and Pharmacy. 1973; 25:271-283
32 Buzzelli G, Moscarella S, Giusti A et al. “A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis”. Int J Clin Pharmacol Ther Toxicol. 1993; 31:456-460
33 Khayyal MT, el-Ghazaly MA, Kenawy SA, Seif-el-Nasr M, Mahran LG, Kafafi YA, Okpanyi SN. “Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination”. Arzneimittelforschung. 2001; 51(7):545-53
34 Khayyal MT, Seif-El-Nasr M, El-Ghazaly MA, Okpanyi SN, Kelber O, Weiser D. “Mechanisms involved in the gastro-protective effect of STW 5 (Iberogast) and its components against ulcers and rebound acidity”. Phytomedicine 2006; 13 Suppl 5:56-66
35 Caceres, A Plantas medicinales de Guatelama. Editorial Universitaria Universidad de san carlos de Guatemala; 1996; Roersch, C. & Van der Hoogte L. Plantas medicinales del surandino del Perú. Ed. Centro de Medicina Andina. Cuzco, Perú., 1988 pp. 104-115; Khare C.P. (Ed.) Indian Medicinal Plants Springer Science, New York, 2007.
36 Dugo P., Mondello L., Cogliandro E., Cavazza A.& Dugo G. “On the genuineness of citrus essential oils. Part LIII. Determination of the composition of the oxygen heterocyclic fraction of lemon essential oils (Citrus limon (L.) Burm. F.) by normal-phase high performance liquid chromatography” Flav & Frag J. 2000; 13(5), 329-334; Naganuma M., Hirose S., Nakayama Y., Nakajima K. & Someya T. “A study of the phototoxicity of lemon oil.” Arch Dermatol Res. 1985; 278(1), 31-6; McHale D. & Sheridan J.B. “Detection of adulterated cold-pressed lemon oil.” Flav. & Frag. J. 1988; 3, 127-133.
37 Khare 2007 Op. Cit.; Manthey JA, Grohmann K. “Phenols in citrus peel by products. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses”. J Agric Food Chem. 2001; 49(7):3268-73; Sultana S, Ali M, Ansari SH, Bagri P. “New 4′-substituted flavones from the fruit peels of Citrus limon (L.) Burm.f.” J Asian Nat Prod Res. 2008;10(11-12):1123-7; Schröder R, Clark CJ, Sharrock K, Hallett IC, MacRae EA. “Pectins from the albedo of immature lemon fruitlets have high water binding capacity”. J Plant Physiol. 2004; 161(4):371-9
38 Christensen CM, Navazesh M. “Anticipatory salivary flow to the sight of different foods”. Appetite. 1984; Dec;5(4):307-15
39 Davis CM, Wintrup, A, Gaetz M, Hickenbottom S, Bauslaught T “Human salivary responses to olfactory stimulation measured by the Surface method”. Canadian Psychology. 1993; 34(2a): 231; Bauslaugh T Non-invasive measurement of parotid salivary activity and stomach motility. University Master Thesis, Simon Fraser University, 1994; Bauslaugh, T e Davis CM “Measuring human salivary activity noninvasively: validation of the surface electrosalivary measure”. Canadian Psychology. 1993; 34(2a): 231
40 Davenport H. W. Physiology of the Digestive Tract, 5th ed., Year Book Medical Publishers, Chicago, 1982
41 Bauslaugh T 1994 Op. Cit.
42 Bonfils, S, Mignon,M. & Roze, C. “Vagal control of gastric secretion” In International Review of Physiology; Gastrointestinal Physiology. University Park Press, Baltimore, 1979; pp. 59-106; Konturek, S. ]., Kwiecien, N., Obtulowicz, W., Mikos, E., Sito, E., Oleksy,J. & Popiela,T. “Cephalic phase of gastric secretion in healthy subjects and duodenal ulcer patients: role of vagal innervation”. Gut. 1978; 20: 875-881; Sharon A. Gidaœ, CK,Rose M. Threatte e Morley R. Kare “Cephalic reflexes: Their role in digestion and possible roles in absorption and metabolism” J. Nutr. 1987; 117: 1191-1196
43 Gasbarrini G, Gallo A, Nicoletti M, Montalto M, Addolorato G. “Evaluation of effects (symptoms and palatability) after ingestion of “Gran Soleil” in healthy volunteers”. J Biol Regul Homeost Agents. 2008; 22(3):201-5; Gallo A, Gasbarrini G, Nicoletti M, Montalto M, Addolorato G. “Evaluation of symptoms and palatability in healthy volunteers after ingestion of an iced dessert by using different flavours”. J Biol Regul Homeost Agents. 2009;23(2):127-31.
44 Tiscornia, O. M. Cresta, M. A. D. Celener D. , Negri, G. Vaccaro, M. I. Waisman H. Bustos Fernandez L. and Dreiling D. A. “Pure pancreatic juice in humans: orange-lemon- juice-induced secretory effects. Comparative analysis with a regular meal, sorbitol, acidified peptone broth and secretin” Journal International Journal of Gastrointestinal Cancer . 1988; 3 (6)
45 Duke, JA Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press, 1992a; Wichtl, M. Teedrogen. Ein Handbuch fur Apotheker und Arzte. Wissenschaftliche Verlagsgesellscharft. mbH Stuttgart, 1984; Williamson, E. M. and Evans, F. J. Potter’s New Cyclopaedia of Botanical Drugs and Preparations, Revised Ed. 1988; Duke, JA. Handbook of biologically active phytochemicals and their activities. Boca Raton, FL. CRC Press, 1992b; Newall, C. A., Anderson, L. A. and Phillipson, J. D. Herbal Medicine – A Guide for Health-care Professionals. The Pharmaceutical Press, London, 1996; Werbach, M. Healing with Food. Harper Collins, New York, 1993.
46 Harborne Jeffery B. and Baxter, H. eds. Phytochemical Dictionary. A Handbook of Bioactive Compounds from Plants. Taylor & Frost, London, 1983; Zebovitz, T. C. Ed. Part VII. Flavor and Fragrance Substances, in Keith L. H. and Walters, D.B., eds. Compendium of Safety Data Sheets for Research and Industrial Chemicals. VCH Publishers, New York, 1989; Newall, Anderson, and Phillipson 1996 Op. Cit.
47 Grieve A Modern Herbal. Penguin, 1984; Nadkarni KM Indian Materia Medica. Popular Prakashan, Bombay, 1976; Rastogi RP, Mehrotra BN Compendium of Indian medicinal plants. Volume I-IV. Central Drug Research Institute, Lucknow, and National Institute of science communication, New Delhi, India, 1994; Rastogi RP, Mehrotra BN Glossary of Indian Medicinal Plants. National Institute of science communication, New Delhi, India, 2002.
48 Duke 1992b Op. Cit.; Buchbauer, G., Jirovetz, L., Nikiforov, A., Remberg, G., Raverdino, V. “Headspace-Analysis and Aroma Compounds of Austrian Hay-Blossoms (Flores Graminis, Graminis Flos) used in Aromatherapy”. J. Ess. Oil Res. 1990; 2: 185-191; Duke (2009) http://www.ars-grin.gov/duke/
49 Pinkas M, Bezanger-Beauquesne L. Les plantes dans la therapeutique moderne 2a edizione, Parigi, Ed Maloine, 1986.
50 Farnsworth ed. NAPRALERT database. Chicago, University of Illinois at Chicago, IL; Brand N. “Foeniculum”. In: Hänsel R, Keller K, Rimpler H, Schneider G, editors. Hagers Handbuch der Pharmazeutischen Praxis, 5th ed. Volume 5: Drogen E-O. Berlin-Heidelberg-New York-London: Springer-Verlag: 1993, 156-81; Forster HB, Niklas H, Lutz S. “Antispasmodic effects of some medicinal plants”. Planta Medica. 1980;, 40:309-319; Weiss RF. Lehrbuch der Phytotherapie, 7th ed. Stuttgart, Hippokrates, 1991; Blumenthal 1998 Op. Cit.
51 Alexandrovich I, Rakovitskaya O, Kolmo E, Sidorova T, Sushunov S. “The effect of fennel (Foeniculum Vulgare) seed oil emulsion in infantile colic: a randomized, placebo-controlled study”. Altern Ther Health Med. 2003; 9:58-61
52 Savino F, Capasso R, Palumeri E, Tarasco V, Locatelli E, Capasso F. “Advances on the effects of the compounds of a phytotherapic agent (ColiMil) on upper gastrointestinal transit in mice”. Minerva Pediatr. 2008; 60(3):285-90
53 Capasso R, Savino F, Capasso F. “Effects of the herbal formulation ColiMil on upper gastrointestinal transit in mice in vivo“. Phytother Res. 2007; 21(10):999-1101; et al. 2008 Op. Cit.
54 Chakurski et al. 1981 Op. Cit.
55 Brand 1993 Op. Cit.; Czygan F-C, Hiller K. “Foeniculi amari fructus – Bitterer Fenchel, Foeniculi dulcis fructus – Süßer Fenchel”. In: Teedrogen und Phytopharmaka. Ein Handbuch für die Praxis auf wissenschaftlicher Grundlage, 4th ed. Stuttgart: Wissenschaftliche Verlagsgesellschaft: 2002, 212-5; Müller-Limmroth W, Fröhlich HH. “Effect of various phytotherapeutic expectorants on mucociliary transport.” Fortschrift für Medizin. 1980; 98:95-101; Blumenthal et al. 1998 Op. Cit.; Hänsel et al. 1993) Op. Cit; Forster, Niklas, Lutz 1980 Op. Cit.
56 Ghelardini C, Galeotti N, Mazzanti G. “Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils”. Planta Med. 2001; 1;67:564-6
57 Haginiwa J, Harada M, Morishita I. “Pharmacological studies on crude drugs VII. Properties of essential oil components of aromatics and their pharmacological effects on mouse intestine.” Yakugaku Zasshi. 1963; 83:624-628; Ostad SN Soodi M, Shariffzadeh M, Khorshidi N, Marzban H “The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study”. Journal of Ethnopharmacology. 2001; 76:299-304
58 Lis-Balchin M, Hart S. “A preliminary study of the effect of essential oils on skeletal and smooth muscle in vitro”. J Ethnopharmacol. 1997; 58:183-7
59 Forster et al. 1980 Op. Cit.
60 Schuster KP. “Intensity and loss of the in situ effect of spasmolytically active drugs, galenic preparations (crude drugs) and galenic drugs in finished dosage form, on isolated gut of guinea-pig and cat” Dissertation, University of Munich, 1971
61 Niiho Y, Takayanagi I, Takagi K. “Effects of a combined stomachic and its ingredients on rabbit stomach motility in situ“. Japanese Journal of Pharmacology. 1977; 27:177-179
62 Vasudevan K, Vembar S, Veeraraghavan K, Haranath PSRK. “Influence of intragastric perfusion of aqueous spice extracts on acid secretion in anesthetized albino rats”. Indian J Gastroenterol. 2000; 19:53-6
63 Platel K, Srinivasan K. “A study of the digestive stimulant action of select spices in experimental rats”. J Food Sci Technol. 2001; 38:358-361
64 Platel, K., Srinivasan, K. Stimulatory influence of select spices on bile secretion in rats. Nutr Res 2000; 20:1493-1503
65 Weizman Z, Alkrinawi S, Goldfarb D, Bitran C. “Efficacy of herbal tea preparation in infantile colic”. J Pediatr. 1993; 122:650-652
66 Vejdani R, Shalmani HR, Mir-Fattahi M, Sajed-Nia F, Abdollahi M, Zali MR, Alizadeh AH, Bahari A, Amin G “The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: a pilot study” Dig Dis Sci. 2006 Aug;51(8):1501-7
67 Itokawa, H., S. Mihashi, K. Watanabe, H. Natsumoto,, and T. Hamanaka. “Studies on the constituents of crude drugs having inhibitory activity against contraction of the ileum caused by histamine or barium chloride (1) screening test for the activity of commercially available crude drugs and the related plant materials”. ShoyakugakuZasshi 1983; 37(3): 223-228; Wagner H, Sprinkmeyer L. “Über die pharmakologische Wirkung von Melissengeist”. DeutscheApotheker Zeitung, 1973, 113:1159-1166; Debelmas AM, Rochat J. “Étude pharmacologique des huiles essentielles. Activité antispasmodique etudiée sur une cinquantaine d’échantillons differents”. Plantes médicinales et Phytothérapie, 1967, 1:23-27; Reiter M, Brandt W. “Relaxant effects on tracheal and ileal smooth muscles of the guinea-pig”. Arzneimittel-Forschung, 1985, 35:408-414.
68 Forster et al. 1980 Op. Cit.
69 Hose et al. “Ontogenic variation of the essential leaf oil of Melissa officinalis L.”. Pharmazie. 1997; 52:247-253
70 Duband, F., Carnat, A. P., Carnat, A., Petitjean-Freytet, C., Clair, G., & Lamaison, J. L. “Aromatic and polyphenolic composition of infused peppermint, Mentha x piperita L.”, Ann Pharm Fr. 1992;, 50 (3):146-55.
71 Reynolds JEF, ed. Martindale, the extra pharmacopoeia, 30th ed. London, Pharmaceutical Press, 1996.
72 Leicester RJ, Hunt RH. “Peppermint oil to reduce colonic spasm during endoscopy”. Lancet. 1982; 2:989.
73 Kingham, J. G. C. “Peppermint oil and colon spasm”, Lancet. 1995;, 346(8981): 986; Sparks MJW et al. “Does peppermint oil relieve spasm during barium enema?” British Journal of Radiology. 1995;, 68:841-843.
74 Hawthorne, M et al. “The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal, and cardiac preparations”. Aliment Pharmacol. Ther. 1988; 2:108-118
75 Kingham 1995 Op. Cit.
76 Dalvi SS et al. “Effect of peppermint oil on gastric emptying in man: a preliminary study using a radiolabelled solid test meal”. Indian Journal of Physiology and Pharmacology, 1991, 35:212-214.
77 Goerg, K. J. & Spilker, T. “Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: a pharmacodynamic study usingsimultaneous determination of gastric and gall-bladder emptying and orocaecal transit time”, Aliment Pharmacol Ther. 2003; 17 (3):445-51.
78 Micklefield, G., Jung, O., Greving, I., & May, B. “Effects of intraduodenal application of peppermint oil (WS(R) 1340) and caraway oil (WS(R) 1520) on gastroduodenal motility in healthy volunteers”, Phytother Res. 2003;, 17 (2): 135- 40.
79 May, B., Kuntz, H. D., Kieser, M., & Kohler, S. “Efficacy of a fixed peppermint oil/caraway oil combination in non-ulcer dyspepsia”, Arzneimittelforschung. 1996; 46(12):1149-53.
80 Freise, J. & Kohler, S. “Peppermint oil-caraway oil fixed combination in non- ulcer dyspepsia– comparison of the effects of enteric preparations”, Pharmazie. 1999; 54(3):210-5.
81 May, B., Kohler, S., & Schneider, B. “Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia”, Alimentary Pharmacology & Therapeutics. 2000; 14(12):1671-1677.
82 Madisch, A., Melderis, H., Mayr, G., Sassin, I., & Hotz, J. “A plant extract and its modified preparation in functional dyspepsia. Results of a double-blind placebo controlled comparative study”, Z Gastroenterol. 2001; 39(7):511-7.
83 Goerg & Spilker 2003 Op. Cit. 2003
84 Coon, J. T. & Ernst, E. “Systematic Review: Herbal medicinal products for non-ulcer dyspepsia”, Alimentary Pharmacology & Therapeutics. 2002; 16(10):1689-1699.
85 Reiter, Brandt 1985 Op. Cit.; Taddei I et al. “Spasmolytic activity of peppermint, sage and rosemary essences and their major constituents”.Fitoterapia. 1988; 59:463-468; Triggle DJ et al. “Peppermint oil as a calcium channel antagonist in intestinal smooth muscle and neuronal preparations”. Gastroenterology. 1988; 94:A465; Taylor BA, Duthie HL, Luscombe DK. “Inhibitory effect of peppermint oil on gastrointestinal smooth muscle”. Gut. 1983; 24:A992. Hills, J. M. & Aaronson, P. I. “The mechanism of action of peppermint oil on gastrointestinal smooth muscle. An analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig”, Gastroenterology. 1991; 101(1):55-65.
86 ESCOP 2003 Op. Cit.; Forster et al. 1980 Op. Cit; Leslie GB. “A pharmacometric evaluation of nine Bio-Strath herbal remedies”. Medita. 1978; 8:3-19; Lallement-Guilbert N, Bézanger-Beauquesne L. “Recherches sur les flavonoides quelques Labiees médicinales (romarin, menthe poivrée, suage officinale)”. Plantes médicinales et Phytothérapie. 1970; 4:92-107.
87 Sigmund CJ, McNally EF. “The action of a carminative on the lower esophageal sphincter”. Gastroenterology, 1969, 56:13-18; Massey, B. T. “Diffuse esophageal spasm: a case for carminatives?”, J Clin Gastroenterol. 2001; 33(1):8-10.
88 Harries N, James KC, Pugh WK. “Antifoaming and carminative actions of volatile oils”. Journal of Clinical Pharmacy. 1978; 2:171-177.
89 Lallement-Guilbert, Bézanger-Beauquesne. 1970 Op. Cit.; Leslie 1978 Op. Cit.; ESCOP 2003 Op. Cit.
90 Goerg & Spilker 2003 Op. Cit. 2003
91 Taylor BA et al. “Mechanism by which peppermint oil exerts its relaxant effect on gastrointestinal smooth muscle”. Journal of Pharmacy and Pharmacology. 1985; 37 (Suppl. 1):104.
92 Heimes K, Hauk F, Verspohl EJ. “Mode of action of peppermint oil and (-)-menthol with respect to 5-HT(3) receptor subtypes: binding studies, cation uptake by receptor channels and contraction of isolated rat ileum”. Phytother Res. 2010 Nov 12. doi: 10.1002/ptr.3316.
93 Bruneton 1995 Op. Cit; Carle, R. and Isaac, O. Z Phytotherapie. 1987; 8, 67; Schilcher, H. Die Kamille: Handbuch fur Arzte, Apotheker und andere Naturwissenschaftler, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1987
94 Stiegelmeyer, H. “Therapie unspezifischer Magenbeschwerden mit Kamillosan.”, Kassenarzt. 1978; 18:3605-3606
95 Weizman et al. 1993 Op. Cit.
96 de la Motte S, Bose-O’Reilly S, Heinisch M, Harrison F. “Double-blind comparison of an apple pectin-chamomile extract preparation with placebo in children with diarrhea” Arzneimittelforschung. 1997; 47:1247-1249
97 Breinlich VJ, Scharnagel K. “Pharmacologic characteristics of the en-yn-dicycloethers from Matricaria chamomilla”. Arzneimittelforschung. 1968; 18:429-431; Mann C, Staba EJ. “The chemistry, pharmacology, and commercial formulations of chamomile”. In: Craker LE, Simon JE, eds. Herbs, Spices, and Medicinal Plants. Recent Advances in Botany, Horticulture, and Pharmacology, Vol. 1. Binghamton, NY: Haworth Press; 1986; Holzl J, Ghassemi N, Hahn B. “Preparation of 14C-spiro ethers by chamomile and their use by an investigation of absorption”. Planta Med. 1986; 52:553; Della Loggia, R., Carle, R., Sosa, S., & Tubaro, A. “Evaluation of the anti- inflammatory activity of Chamomile preparations”, Planta Medica. 1990; 56(6):657-658; Cinco, M., Banfi, E., Tubaro, A., & Dellaloggia “A microbiological survey on the activity of a hydroalcoholic extract of camomile”, International Journal of Crude Drug Research. 1983; 21(4)_145-151.
98 Redaelli,C., et al. J.Chrom. 1981; 209, 110.
99 Achterrath-Tuckermann, U., Kunde, R., Flaskamp, E., Isaac, O., & Thiemer, K. “Pharmacological investigations with compounds of chamomile. V. Investigations on the spasmolytic effect of compounds of chamomile and Kamillosan on the isolated guinea pig ileum”, Planta Med. 1980; 39(1):38-50; Lang W, Schwandt K. “Untersuchung über die glykosidischen Bestandteile der Kamille.” Deutsche Apotheker Zeitung, 1957; 97:149-151; Redaelli C, Formentini L, Santaniello E “HPLC-determination of apigenin in natural samples, chamomile extracts and blood serum [Poster]” Int. Res. Congress on natural Products and Medicinal Agents. 1980; Strasbourg, cited in Carle, R. and Isaac, O. Z Phytotherapie. 1987; 8, 67
100 Achterrath-Tuckermann, et al. 1980 Op. Cit.
101 Maschi O, Cero ED, Galli GV, Caruso D, Bosisio E, Dell’Agli M. “Inhibition of human cAMP-phosphodiesterase as a mechanism of the spasmolytic effect of Matricaria recutita L.” J Agric Food Chem. 2008; 9;56(13):5015-20
102 Pasechnik, I. K. “[Choleretic action of Matricaria officinalis]”, Farmakol Toksikol. 1966; 29(4):468-9.
103 Gupta, M.P. 270 Plantas Medicinales Iberoamericanas, Talleres de Editorial Presencia Ltda., Bogota ICMR, 1995; Indian Council of Medical Research Medicinal Plants of India, Vol. 2, Indian Council of Medical Research, Cambridge Printing Works, New Delhi, 1987; Arvigo, R. and Balick, M., Rain Forest Remedies — One Hundred Healing Herbs of Belize, Lotus Press, Twin Lakes, 1993; Germosén-Robineau, L., Ed. Farmacopea Vegetal Caribeña, Enda-caribe, Tramil, Ediciones, 1997; Duke, J.A. and Vasquez Martinez, R. Amazonian Ethnobotanical Dictionary, CRC Press, Boca Raton, 1994
104 Duke and Vasquez Martinez 1994 Op. Cit.; Arvigo and Balick 1993 Op. Cit.; Peirce, A. The APhA Practical Guide to Natural Medicines, Stonesong Press Book, Wm. Morrow & Co., Inc., New York, 1999; Gupta 1995 Op. Cit.; ICMR – Indian Council of Medical Research Medicinal Plants of India, Vol. 2, Indian Council of Medical Research, Cambridge Printing Works, New Delhi, 1987; Germosén-Robineau 1997 Op. Cit.; Duke, J.A. and Ayensu, E.S. Medicinal Plants of China, 2 vols., Reference Publications, Algonac, 1985.
105 Caceres 1996 Op. Cit.
106 De Vincenzi, M. et al. “Monographs on botanical flavouring substances used in foods. Part IV”. Fitoterapia. 1994; 66(3):206-207.
107 Williamson, Evans 1988 Op. Cit.; Caceres 1996 Op. Cit.; Torrent Martí, M.T. “Algunos aspectos farmacognósticos y farmacodinámicos de Lippia citriodora H.B.K.” Revista de la Real Academia de Farmacia de Barcelona. 1985a; (14): 39-55; Carnat A., Carnat A.-P., Chavignon O., Heitz A., Wylde R., Lamaison J.-L., Planta Med. 1995;, 61, 490; Lamaison, J.L. et al. “Le verbascoside, compose phenolique majeur del feuilles de frene (Fraxinus excelsior) et de verveine (Aloysia triphylla)”. Pl. Medic. et Phytother. 1993; 26(3):225-233; Skaltsa. H. e G. Shammas. “Flavonoids from Lippia citriodora“. Planta Medica. 1988; (5):465; Valentão P., Andrade P. B., Areias F., Ferreres F., Seabra R. M. J. Agric. Food Chem. 1999; 47, 4579–4582; Andrade P. B., Seabra R. M., Valentão P., Areias F. J. Liq. Chrom. & Rel. Technol. 1998; 21, 2813-2820.
108 Newall, Anderson, Phillipson 1996 Op. Cit.
109 Baudi, O. Plantas Medicinales existentes en Venezuela y Latinoamérica. Editorial América. Caracas, 1987; Torrent Martí, M.T. “Acción farmacológica de algunas esencias de origen biológico”. Revista de la Real Academia de Farmacia de Barcelona. 1985; (1): 43-56; Torrent Martí 1985a Op. Cit.; Newall, Anderson., Phillipson 1996 Op. Cit.; Duke 1985 Op. Cit.; Fleming 1998 Op. Cit.
110 Duke 1985 Op. Cit.; List, P.H. and Hohammer, L., Hager’s Handbuch der Pharmazeutischen Praxis, Vols. 2-6, Springer-Verlag, Berlin, 1969-1979
111 García, B. H. Flora Medicinal de Colombia. Editorial Tercer Mundo. Editores Bogotá. Colombia. 1982; Newall, Anderson., Phillipson 1996 Op. Cit.; Jain, S.K. and deFillips, R., Medicinal Plants of India, 2 vols., Reference Publications, Algonac, 1991; Council of Scientific and Industrial Research (CSIR) The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products, Revised, Vols. 1-3, CSIR, New Delhi, 1992
112 Caceres 1996 Op. Cit.; Newall, Anderson., Phillipson 1996 Op. Cit.
113 Dabroy L. “Confirmacion de la actividad antibacteriana de algunas especies del genere Lippia contra bacterias que causan infeccion respiratoria” (Tesis) Giatemala Fac CCQQ y Farm, 1994; Arteche A Fitoterapia. Vademecum de prescripcion. Bilbao, CITA, 1994
114 Williamson and Evans 1988 Op. Cit.
115 Ragone MI, Sella M, Conforti P, Volonté MG, Consolini AE. “The spasmolytic effect of Aloysia citriodora, Palau (South American cedrón) is partially due to its vitexin but not isovitexin on rat duodenums”. J Ethnopharmacol. 2007; 113(2):258-66.
116 Caceres 1996 Op. Cit.
117 Yeung Him-Che Handbook of Chinese Herbs and Formulas, Institute of Chinese Medicine, Los Angeles, 1985
118 Jodral MM Illicium, Pimpinella, and Foeniculum CRC Press, Boca Raton, 2004
119 Reiter, M., and W. Brandt. “Relaxant effects on tracheal and ileal smooth muscles of the guinea pig”. Arzneim- Forsch 1985; 35(1): 408-414.; Albuquerque AA, Sorenson AL, Leal-Cardoso JH. “Effects of essential oil of Croton zehntneri, and of anethole and estragole on skeletal muscles”. Journal of Ethnopharmacology. 1995; 49:41-49
120 Melzig, M e Teuschen, E. “Investigations of the influence of essential oil and their main components on the adenosine uptake by cultivated endothelial cells”. Planta Medica. 1991; 57(1):41-42; Tirapelli CR, de Andrade CR, Cassano AO, D Souza FA, Ambrosio SR, da Costa FB, de Oliveira AM. “Antispasmodic and relaxant effects of the hidroalcoholic extract of Pimpinella anisum (Apiaceae) on rat anococcygeus smooth muscle”. J Ethnopharmacol. 2007; 1;110(1):23-9
121 Peirce, A., The APhA Practical Guide to Natural Medicines, Stonesong Press Book, Wm. Morrow & Co., Inc., New York, 1999; Duke 1985 Op. Cit.; Leung, A.Y. and Foster, S., Encyclopedia of Common Natural Ingredients, 2nd ed., John Wiley & Sons, New York, 1995; Gruenwald, J. et al., PDR for Herbal Medicines, 2nd ed., Medical Economics Co., Montvale, NJ, 2000; Blumenthal et al. 1998 Op. Cit.
122 Peirce 1999 Op. Cit.; Duke 1985 Op. Cit.; Williamson and Evans 1988 Op. Cit.; Gruenwald et al. 2000 Op. Cit.
123 Peirce 1999 Op. Cit.; Duke 1985 Op. Cit.; Gruenwald et al. 2000 Op. Cit.; Blumenthal et al. 1998 Op. Cit.
124 ASEAN Standard of ASEAN herbal medicine, Vol. I. Jakarta, ASEAN Countries, 1993; Farnsworth Op. Cit.; Blumenthal et al. 1998 Op. Cit.; Bisset NG. Max Wichtl’s herbal drugs & phytopharmaceuticals. Boca Raton, FL, CRC Press, 1994; Ghazanfar SA. Handbook of Arabian medicinal plants. Boca Raton, FL, CRC Press, 1994; Chang HM, But PPH, eds. Pharmacology and applications of Chinese materia medica, Vol. 1. Singapore, World Scientific Publishing, 1986.
125 ASEAN 1993 Op. Cit.; Awang, D. V. C. “Ginger”, Canadian Pharmaceutical Journal. 1992; 125(7):309-311; Yoshikawa, M., Chatani, N., Hatakeyama, S., Nishino, Y., Yamahara, J., & Murakami, N. “Crude drug processing by far-infrared treatment. II. Chemical fluctuation of the constituents during the drying of Zingiberis Rhizoma”, Yakugaku Zasshi. 1993; 113(10):712-717.
126 Platel, K. & Srinivasan, K. “Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats” International Journal of Food Sciences & Nutrition. 1996; 47(1):55-59; Platel, K., and K. Srinivasan. “Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats”. Nahrung 2000; 44(1): 42-46; Yamahara, J., Miki, K., & Chisaka, T. “Cholagogic effect of ginger and its active constituents” Journal of Ethnopharmacology. 1985; 13(2):217-225..
127 Yamahara et al. 1985 Op. Cit..
128 Platel and Srinivasan 1996 Op. Cit.; Platel and Srinivasan 2000 Op. Cit.
129 Reiter and Brandt 1985 Op. Cit..
130 Itokawa et al. 1983 Op. Cit.
131 Kato, M., J. Nagao, M. Hayashi, and E. Hayashi. “Pharmacological studies on saiko-prescriptions. I. Effects of saiko- prescriptions on the isolated smooth muscles”. Yakugaku Zasshi 1982; 102: 371-380.
132 Hung, N. D., I. K. Chang, H. C. Jung, and N. J. Kim. “Studies on the efficacy of combined preparation of crude drugs (XII)”. Korean J Pharmacog 1983; 14(1): 9-16.
133 Gupta, Y. K. & Sharma, M. “Reversal of pyrogallol-induced delay in gastric emptying in rats by ginger (Zingiber officinale)” Methods & Findings in Experimental & Clinical Pharmacology. 2001; 23(9):501-503; Micklefield, G. H., Redeker, Y., Meister, V., Jung, O., Greving, I., & May, B. “Effects of ginger on gastroduodenal motility”, Int J Clin Pharmacol Ther. 1999; 37(7):341-6; Yamahara, J., Huang, Q., Li, Y., Xu, L., & Fujimura, H. “Gastrointestinal motility enhancing effect of ginger and its active constituents”, Chemical & Pharmaceutical Bulletin. 1990; 38(2):430-431.
134 Shibata, C., I. Sasaki, H. Naito, T. Ueno, and S. Matsuno. “The herbal medicine Dai-Kenchu-Tou stimulates upper gut motility through cholinergic and 5-hydroxytryptamine 3 receptors in conscious dogs”. Surgery 1999; 126(5): 918-924.
135 Stewart, J. J., Wood, M. J., Wood, C. D., & Mims, M. E. “Effects of ginger on motion sickness susceptibility and gastric function” Pharmacology. 1991; 42(2):111-120; Phillips, S., Hutchinson, S., & Ruggier, R. “Zingiber officinale does not affect gastric emptying rate: A randomised, placebo-controlled, crossover trial”, Anaesthesia. 1993; 48(5):393-395.
136 Ghayur MN, Gilani “Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders”. AH Dig Dis Sci. 2005; 50(10):1889-97; Ghayur MN, Gilani AH. “Species differences in the prokinetic effects of ginger”. Int J Food Sci Nutr. 2006; 57(1-2):65-73; Ghayur MN, Khan AH, Gilani AH. “Ginger facilitates cholinergic activity possibly due to blockade of muscarinic autoreceptors in rat stomach fundus”. Pak J Pharm Sci. 2007; 20(3):231-5.
137 Huang, Q. R., Iwamoto, M., Aoki, S., Tanaka, N., Tajima, K., Yamahara, J., Takaishi, Y., Yoshida, M., Tomimatsu, T., & Tamai, Y. “Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger”, Chem Pharm Bull . 1991; 39(2):397-9; Yamahara J et al. “Inhibition of cytotoxic drug-induced vomiting in suncus by a ginger constituent”. Journal of ethnopharmacology. 1989; 27:353-355. Mustafa T, Srivastava KC, Jensen KB. “Drug development report 9. Pharmacology of ginger, Zingiber officinale“. Journal of drug development, 1993, 6:25-39; Yamahara, et al. 1990 Op. Cit.
138 Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. “Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum”. Eur J Pharmacol. 2005;530(1-2):136-43
139 Nievergelt A, Huonker P, Schoop R, Altmann KH, Gertsch J. “Identification of serotonin 5-HT1A receptor partial agonists in ginger”. Bioorg Med Chem. 2010; 18(9):3345-51
140 Huang et al. 1991 Op. Cit.; Mustafa, Srivastava, Jensen 1993 Op. Cit.; Yamahara, J., Huang, Q., Li, Y., Xu, L., & Fujimura, H. “Gastrointestinal motility enhancing effect of ginger and its active constituents”. Chemical & Pharmaceutical Bulletin. 1990; 38(2):430-431.
141 Abdel-Aziz et al. 2006 Op. Cit.
142 Meyer, K., Schwartz, J., Crater, D., & Keyes, B. “Zingiber officinale (ginger) used to prevent 8-Mop associated nausea” Dermatol Nurs. 1995; 7(4):242-4. Arfeen Z, Owen H, Plummer JL, Ilsley AH, Sorby-Adams RAC, Doecke CJ. “A double-blind randomized controlled trial of ginger for the prevention of postoperative nausea and vomiting.” Anaesth Intens Care 1995; 23:449-452; Phillips S, Ruggier R, Hutchinson SE. “Zingiber officinale (ginger)–an antiemetic for day case surgery”. Anaesthesia 1993; 48:715-717; Fisher-Rasmussen W, Kjaer SK, Dahl C, Asping U. “Ginger treatment of hyperemesis gravidarum“. Eur J Obstet Gynecol Reprod Biol 1990; 38:19-24; Wood CD, Manno JE, Wood MJ, Manno BR, Mims ME. “Comparison of efficacy of ginger with various antimotion sickness drugs”. Clin Res Pr Drug Regul Aff. 1988; 6(2):129-136; Bone ME, Wilkinson DJ, Young JR, McNeil J, Charlton S. “Ginger root–a newantiemetic”. Anesthesia 1990; 45:669-671; Ernst, E. & Pittler, M. H. “Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials” Br J Anaesth. 2000; 84(3):367- 71; Visalyaputra S, Petchpaisit N, Somcharoen K, Choavaratana R. “The efficacy ofginger root in the prevention of postoperative nausea and vomiting after outpatient gynaecological laproscopy”. Anaesthesia 1998; 53:486-510; Vutyavanich T, Kraisarin T, Ruangsri RA. “Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial”. Obstet Gynecol 2001; 97(4):577-582; Grontved A, Brask T, Kambskard J, Hentzer E. “Ginger root against seasickness”. Acta Otolaryngol 1988;105:45-49; Stewart et al. Op. Cit.; 1991.
143 Ernst, Pittler 2000 Op. Cit.
144 Jewell, D. & Young, G. “Interventions for nausea and vomiting in early pregnancy”, Cochrane Database Syst Rev. 2002; 1, CD000145.
145 Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. “A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia”. Clin Ther 1993; 15: 510-25; Epstein JB, Marcoe JH. “Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia”. Oral Surg Oral Med Oral Pathol 1994; 77:135-40; Sicuteri F, Fusco BBM, Marabini S, et al. “Beneficial effect of capsaicin application to the nasal mucosa in cluster headache”. Clin J Pain 1989; 5: 49-53. The Capsaicin Study Group. “Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, doubleblind, vehicle-controlled study”. Arch Intern Med 1991; 151:2225-9; Barbanti G, Maggi CA, Beneforti P, Baroldi P, Turini D. “Relief of pain following intravesical capsaicin in patients with hypersensitive disorders of the lower urinary tract”. Br J Urol 1993; 71: 686-91
146 Lynn B. “Capsaicin: actions on nociceptive C-fibres and therapeutic potential”. Pain 1990; 41: 61-9; Holzer P. “Capsaicin: cellular targets, mechanism of action, and selectivity for thin sensory neurons”. Pharmacol Rev 1991; 43: 143-201; Mayer EA, Gebhart GF. “Basic and clinical aspects of visceral hyperalgesia”. Gastroenterology 1994; 107: 271-93
147 Rodriguez-Stanley S, Collings KL, Robinson M, Owen W, Miner JR. “The effects of capsaicin on reflux, gastric emptying and dyspepsia”. Aliment Pharmacol Ther 2000; 14: 129-34;
148 Bortolotti M, Coccia G, Grossi G, Miglioli M “The treatment of functional dyspepsia with red pepper” Aliment Pharmacol Ther 2002; 16: 1075-1082
149 Debreceni A, Abdel-Salam OM, Juricskay I, Szolesanyi J,Mozsik G. “Capsaicin increases gastric rate in healthy human subjects measured by 13C-labeled octanoic acid breath test”. J Physiol (Paris) 1999; 93: 455-60
150 Lippe ITH, Pabst MA, Holzer P. “Intragastric capsaicin enhances rat gastric acid elimination and mucosal blood flow by afferent nerve stimulation”. Br J Pharmacol 1989; 96: 91-100
151 Alfoldi P, Obal F, Toth E, Hideg J. “Capsaicin pretreatment reduces the gastric acid secretion elicited by histamine but does not affect the responses to carbachol and pentagastrin”. Eur J Pharmacol 1986; 123: 321-7; Rodriguez-Stanley et al. 2000 Op. Cit.; 14: 129-34; Park HJ, Na SK, Lee SI, Kang JK, Park IS. “The effect of red pepper and capsaicin on gastric emptying in human volunteers”. Gastroenterology 1998; 114: G3361
152 Alfoldi et al. 1986 Op. Cit.; Mozsik G, Debreceni A, Abdel-Salam OM, et al. “Small doses of capsaicin given intragastrically inhibit gastric basal acid secretion in healthy human subjects”. J Physiol (Paris) 1999; 93: 433-6.; Gonzalez R, Dunkel R, Koletzko B, Schusdziarra V, Allesher HD. “Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers”. Dig Dis Sci 1998; 43: 11 165-71; Vazquez-Olivencia W, Shah P, Pitchumoni CS. “The effect of red and black pepper on orocecal transit time”. J Am Coll Nutr 1992; 11: 228-31; Horowitz M, Wishart J, Maddox A, Russo A. “The effect of chilli on gastrointestinal transit”. J Gastroenterol Hepatol 1992; 7: 52-6
153 Reinbach HC, Smeets A, Martinussen T, Møller P, Westerterp-Plantenga MS. “Effects of capsaicin, green tea and CH-19 sweet pepper on appetite and energy intake in humans in negative and positive energy balance”. Clin Nutr. 2009; 28(3):260-5
154 Aruoma OI, Hayashi Y, Marotta F, Mantello P, Rachmilewitz E, Montagnier L. “Applications and bioefficacy of the functional food supplement fermented papaya preparation”. Toxicology. 2010; 278(1):6-16. Epub 2010 Sep 24.
155 Duke et al. 2002 Op. Cit.; Caceres 1996 Op. Cit.
156 Williamson 2002 Op. Cit.